In A First, Alzheimer's Blood Test Available in US From June
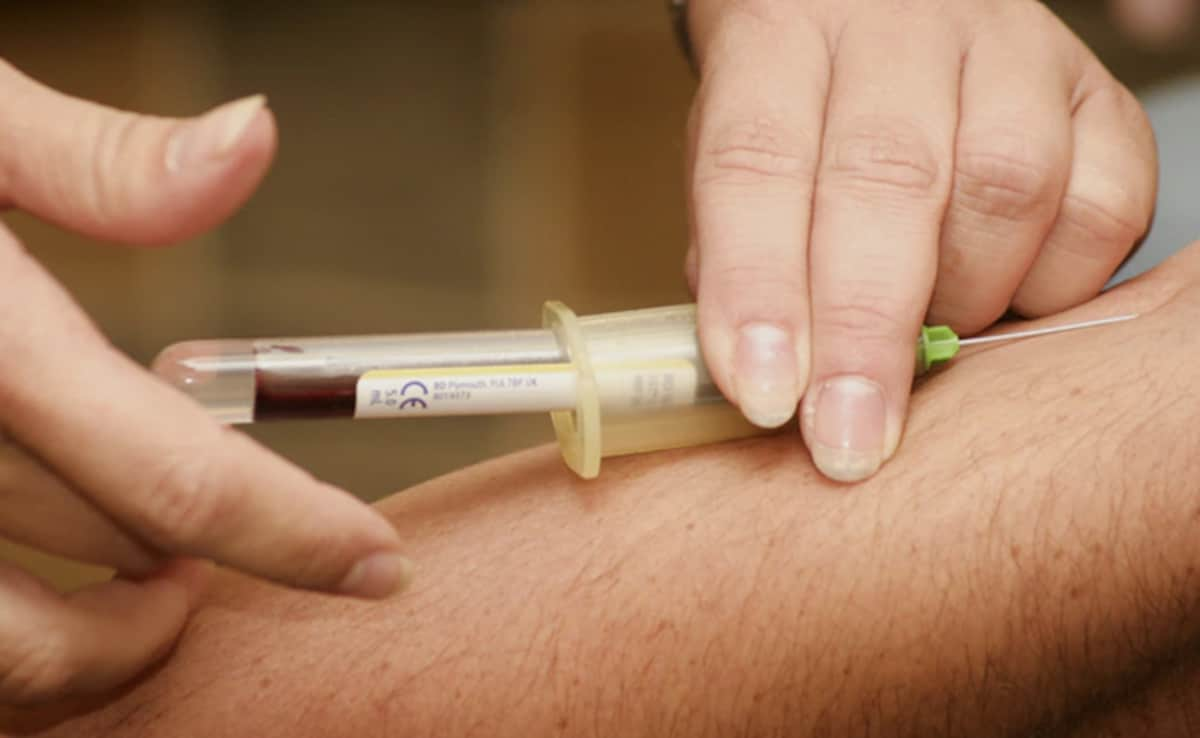
The first blood test to help diagnose Alzheimer’s disease will be available from late June in the US following regulatory clearance for its use last week, according to the Japanese company behind the innovation.
The test will initially be available at about 50 American research institutes and hospitals that specialise in Alzheimer’s disease, Goki Ishikawa, head of Fujirebio Holdings Inc., a unit of Japan’s H.U. Group Holdings Inc., said in an interview Tuesday. It’s partnering with bigger rivals like Beckman Coulter Inc. to help develop and manufacture their products, he said.
The Food and Drug Administration cleared the blood test to help diagnose Alzheimer’s disease last week, potentially making it easier to find and treat patients with the memory-robbing disease that affects nearly 7 million Americans.
The test was cleared for use in people at least 55 years old and who exhibit signs of the disease. It is designed to detect amyloid, a protein that can build up in the brain and is a hallmark of Alzheimer’s, the most common form of dementia in the elderly.
The process, which takes roughly 30 minutes from drawing the blood to diagnosis, will be available to patients at a fraction of the cost of a PET scan. The method is viewed as a critical step in making new types of Alzheimer’s treatments widely accessible.
Until now, patients typically have to get a specialised PET scan to detect amyloid in their brains or undergo cerebrospinal fluid tests, both of which are more expensive and invasive. The lack of quick and easy tests has until now slowed the rollout of new Alzheimer’s drugs like Eisai Co. and Biogen Inc.’s Leqembi and Eli Lilly & Co.’s Kisunla.
The company plans to file data to seek approvals in Japan as early as August and Europe within this year, Ishikawa said. In China, Fujirebio’s undisclosed partner will probably submit data to regulators next year, he added. The company’s partner in India, Agappe Diagnostics Ltd., has already filed data to seek clearance in the country, according to Ishikawa.
“We have a presence in Japan but that’s not necessarily the case in markets overseas,” said Ishikawa. “We can’t get the market shares by ourselves, but if we supply the raw materials to partners, we can benefit through them.”






